How to improve your knee pain during arthroscopy.
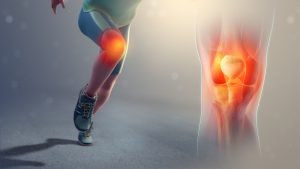
This study examines the injection of leukocyte poor platelet rich plasma during an arthroscopy for knee degeneration. They compared standard knee arthroscopy to arthroscopy with the injection of platelet rich plasma.
Blood consists of plasma (the clear fluid the cells are floating in), white blood cells, red blood cells and platelets. Leukocyte poor platelet rich plasma is prepared by removing the red blood cells, plasma and minimizing the white blood cells. White blood cells cause inflammation. Platelets have many growth and healing factors, but their function is not completely understood.
The conclusion of the study was that leukocyte poor platelet rich plasma injection during arthroscopy showed benefits in pain reduction and increased function at 6 months but no significant benefit at 12 months. More studies are needed.
The Research
Arch Orthop Trauma Surg. 2015 Jul;135(7):971-7. doi: 10.1007/s00402-015-2227-5. Epub 2015 May 10.
Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial.
Duif C1, Vogel T, Topcuoglu F, Spyrou G, von Schulze Pellengahr C, Lahner M.
Abstract
INTRODUCTION:
We aimed to identify the effects of intraoperative applied leukocyte-poor platelet-rich plasma (LP-PRP) during knee arthroscopy for degenerative lesions involving pain, function and quality of life.
METHODS:
We performed a randomized controlled, double-blind trial (RCT) including 58 patients for arthroscopic knee surgery for cartilage or meniscal degeneration with allocation into the LP-PRP (n = 24) or control group (n = 34). During arthroscopy, LP-PRP was injected intra-articular in the intervention group. At baseline, 6 weeks, 6 months and 12 months pain, function, and life quality were assessed.
RESULTS:
91 % of enrolled patients were available for 12 months follow-up. Pain was significantly lower in the LP-PRP group (VAS 0.9. vs. 2.3) at 6 (p = 0.008) but not at 12 months (VAS 1.0 vs. 1.6, p = 0.063). LP-PRP application improved the Lysholm Score at 6 (77.5 vs. 65.6, p = 0.033) and 12 months (83.2 vs.70.0, p = 0.007). Assessment of life quality (SF-36) concerning the physical component summary was significantly higher at 6 weeks (33.9 vs. 25.6, p = 0.001) and 6 months (29.9 vs. 27.1, p = 0.027) in the LP-PRP group but equal at 1 year (31.4 vs. 30.1, p = 0.438).
CONCLUSIONS:
Intraoperative application of LP-PRP may enhance pain reduction and gain of knee function within 6-12 months compared to arthroscopy alone.
LEVEL OF EVIDENCE:
II, randomized controlled clinical trial with reduced power. CLINICALTRIALS.
GOV IDENTIFIER:
NCT02189408.